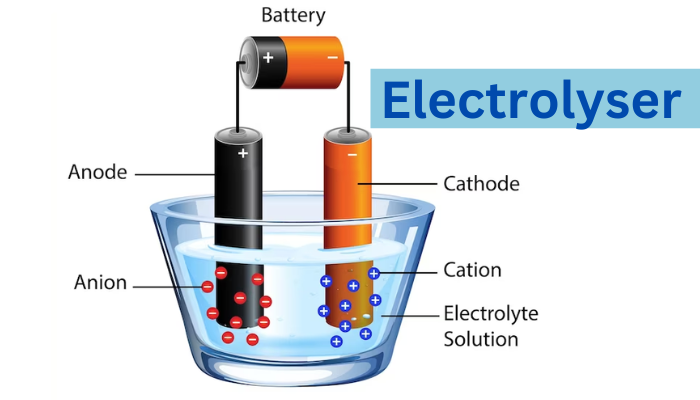
The Electrolyser is a device capable of separating the hydrogen and oxygen molecules that makeup water using electricity and a chemical reaction called electrolysis to produce hydrogen. A decarbonized economy might be built upon hydrogen created in an environmentally friendly manner, which doesn’t release carbon dioxide into the atmosphere.
Hydrogen Generation
Since hydrogen is the most prevalent element in the cosmos, it can serve as the ideal fuel. However, this is not the only factor at play; when hydrogen is consumed, water vapor rather than carbon dioxide is created. By doing this, it would significantly cut down on the emissions that cause the greenhouse effect and global warming.
The problem is that to produce hydrogen, electrical energy is required, and emissions would be produced if this energy were to be derived from fossil fuels. In contrast, the creation of “green hydrogen” relies on the utilisation of renewable energy sources to drive the electrolysis procedure, which creates hydrogen from water. An electrolyser is a device in charge of this procedure.
Electrolyser : What Is It & How Does It Work? Electrolysis
To separate water molecules into their oxygen and hydrogen atoms, a device known as an electrolyser is used. Because of how unstable their bonds are, the two elements must break through a procedure known as electrolysis, which requires electrical energy. For hydrogen to be used in industries and for hydrogen fuel cells to be used, efficient electrolysers will be essential.
Workings of an Electrolyser
In 1800, electrolysis was first identified. Following Alessandro Volta’s development of the electric battery in the same year, several chemists experimented with joining their poles in a water container. They found that the current moved through the water and that the electrodes separated hydrogen from oxygen.
An electrode stack with a membrane separating them makes up an electrolyser, to which high voltage and current are supplied. As a result, the water develops an electric current that leads it to separate into its constituent parts, hydrogen, and oxygen. Pumps, power electronics, a gas separator, and other auxiliary parts like storage tanks are also included in the entire system.
The oxygen produced concurrently is released into the atmosphere or, in some situations, can be saved for later use as a medicinal or industrial gas. For usage in industry or hydrogen fuel cells, which can power vehicles like trains, ships, and even aircraft, the hydrogen is kept as a compressed gas or liquefied.
Electrolyser Types
Depending on their size and purpose, electrolysers today come in a variety of varieties. The most frequently employed are:
Alkaline electrolyser
They combine water and a liquid electrolyte solution, such as potassium or sodium hydroxide. A cell with an anode, a cathode, and a membrane produce hydrogen. To increase the amount of hydrogen and oxygen produced at once, the cells are typically put together in series. Hydroxide ions flow through the electrolyte from the cathode to the anode of each cell when current is delivered to the electrolysis cell stack, producing hydrogen gas bubbles on the cathode side of the electrolyser and oxygen gas bubbles at the anode. They have been in use for more than a century and do not require noble metals as a catalyst, but they are large pieces of apparatus that produce hydrogen with a medium purity and are not very versatile in their application.
Proton Exchange Membrane (PEM) Electrolyser
Proton exchange membranes and solid polymer electrolytes are used in PEM electrolysers. Water splits into hydrogen and oxygen when current is added to the battery, and the hydrogen protons then flow through the membrane to generate hydrogen gas on the cathode side. Because they create very pure hydrogen and are simple to cool, they are the most often used. They are compact, create high-purity hydrogen, and are best adapted to match the variability of renewable sources. However, because they utilise precious metals as catalysts, they are a little more expensive.
Solid oxide electrolysis cell (SOEC)
Compared to PEMs and alkaline electrolysers, SOECs have the potential to be significantly more efficient because they operate at higher temperatures. A solid ceramic substance is used as the electrolyte in a procedure known as high-temperature electrolysis (HTE) or steam electrolysis. At the cathode, water and electrons from the external circuit mix to produce hydrogen gas and negatively charged ions. Then, oxygen travels through the slidable ceramic membrane before reacting at the anode to produce oxygen gas and produce electrons for the external circuit. They are less advanced technologically than the previous group.
IEA Report Aspects (Publised in Sep 2022)
Although SOEC and anion exchange membrane technologies are still in their infancy, alkaline and PEM technologies are currently accessible commercially.
Electrolysers for alkaline and PEM are already marketed. A more established technique with a lengthy history of use in the chlor-alkali sector is alkaline electrolysers. Both technologies, however, are at the same technology readiness level (TRL9) for the specific production of hydrogen since they are both commercially available, but they still need policy support and advancements to remain competitive with more established hydrogen production methods based on unabated fossil fuels. Shortly, it appears that alkaline designs will outsell PEM electrolysers in terms of market share due to the volume and size of projects now in development.
A technology called SOEC electrolysis is currently being tested. In a Neste refinery in the Netherlands, Sunfire is constructing the largest SOEC electrolyser in the world (2.6 MW), which is planned to start operating at the end of 2022. There are also larger-scale projects being worked on, some of which are fairly far along. The Norsk e-fuel project stated in February 2022 that the first phase of its project to generate 12.5 million liters of synthetic kerosene annually with an expected SOEC electrolyser size of 25 MW will begin construction in 2023.
Global electrolysis capacity could exceed 1 GW towards the end of 2022
To create chlorine and sodium hydroxide, electrolysers are a commonly utilised technology in the chlori-alkaline sector. The installed electrolysis capacity has surpassed 20 GW in this industry. But until the late 2010s, when things started to pick up, the deployment of electrolysers for specific hydrogen production was slow. Around 300 MW of water electrolysis capacity have been constructed worldwide as of the end of 2020. In 2021, annual capacity additions increased significantly, making it the year with the largest deployment in the historical series. More than 200 MW of electrolysis capacity went into operation, a threefold increase from the previous record year, and the total installed capacity reached more than 500 MW, an increase of nearly 70% from 2020.
 Source: IEA Report Data
Source: IEA Report DataAccording to the pipeline of projects now in development, the overall capacity of electrolysis worldwide might virtually triple from 2021 to the end of 2022, reaching roughly 1.4 GW. If projects, which are primarily centered in Europe, China, and Australia, are completed on schedule, it might have increased tenfold by 2023. Global electrolysis capacity could reach 134-240 GW in 2030 if every project now in the works is completed. With around 30% of the total capacity each, Europe and Australia dominate the market. Latin America follows with more than 10% of the announced projects.
In comparison to 2021, when the total capacity of projects in the pipeline intending to be operational by 2030 was 54-90 GW, this represents a huge rise. However, the project pipeline needs to grow up considerably more quickly to keep up with the Net Zero Scenario, which calls for installing more than 700 GW of electrolysers worldwide by 2030.
We At GH2 Solar
GH2 Solar is a technology-oriented company that has vast experience in executing oil and refineries solar rooftop & large-scale utility projects across India. Being already experienced in the renewable sector, now we are working on the development of “Green Hydrogen” & HAAS (Hydrogen as a Service). If you are planning to adopt Green Hydrogen, you can connect with GH2 Solar to get all information about the same.
For more information, please give us a call at 1800-102-8685